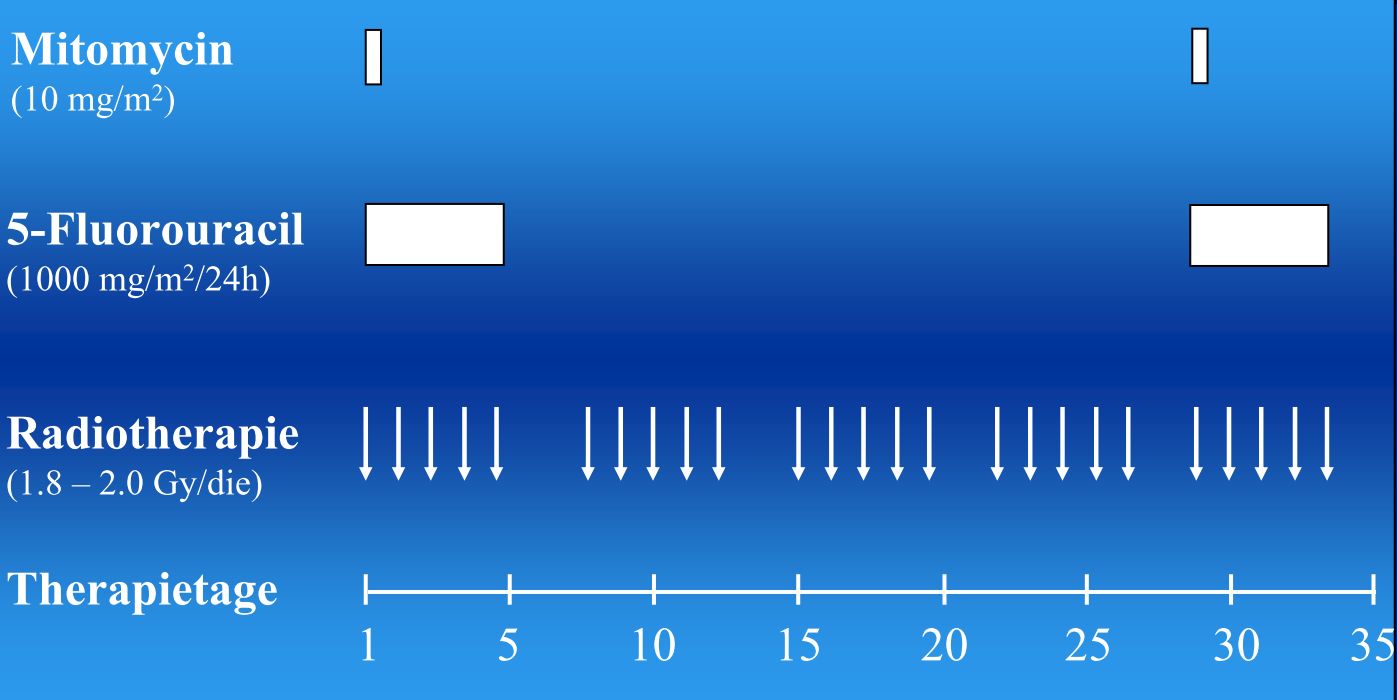
Nach einem Vortrag von Grabenbauer (2003).
| Tag | Zeit | Zytostatikum | Dosis | Applikation | Verdünnung | Bemerkungen |
| 1 | 0:00 | Mitomycin | 15 mg/qm | Bolus i.v. | unverdünnt | |
| 1 | 0:45 | 5-FU | 1000 mg/qm | 22h i.v. | NaCa | |
| 2-4 | 0:00 | 5-FU | 1000 mg/qm | 22h i.v. | NaCa | |
| 29-32 | 0:00 | 5-FU | 1000 mg/qm | 22h i.v. | NaCa | ohne Mitomycin? |
primäre Radiochemotherapie
| Quelle | Fälle | Bestrahlung | Chemotherapie | Lokal - Rezidive | Colostomie | Überleben | Bemerkungen |
| EORTC 22861(1) | 110 | RT 45Gy + 15–20 Gy Boost | - | 48% | 32% | 56% | Radiochemotherapie besser als Radiotherapie allein. |
| RCT 45Gy + 15–20 Gy Boost | 5-FU-MMC | 29% | ? | 56% | |||
| UKCCCR-ACT I (2, 3) | 577 | 45Gy, ggf. 15–25 Gy Boost | - | 54% | ? | ? | Radiochemotherapie besser als Radiotherapie allein. |
| 45Gy, ggf. 15–25 Gy Boost | 5-FU-MMC | 29% | ? | ? | |||
| RTOG 87–04 (4) | 291 | RT 45–50,4 Gy | 5-FU | ? | 22% | 71% | 5-FU-MMC besser als 5-FU allein |
| 45–50,4 Gy | 5-FU - MMC | ? | 9% | 78% | |||
| RTOG 98–11 (5, 6) | 649 | 45–59 Gy | 5-FU - MMC | 21% | 33% | 79% | Kein Unterschied zwischen 5-FU-Cisplatin und 5-FU-MMC |
| 45–59 Gy | 5-FU - Cisplatin | 27% | 41% | 71% | |||
| ACT II (7) | 940 | 50,4 Gy | 5-FU - MMC | ? | 11% | 22% | |
| 50,4 Gy | 5-FU - Cisplatin | ? | 13% | 23% | |||
| UNICANCER ACCORD 03 (8) | 307 | 45 Gy + 15 Gy Boost | 5-FU - Cisplatin | 4,9% | 4,1% | ? | Höhere Boost-Dosis verbessert das Ergebnis nicht. |
| 45 Gy + 20–25 Gy Boost | 5-FU - Cisplatin | 4,9% | ? | ? |
Quellen
Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups.
J Clin Oncol 1997; 15: 2040–9
2. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research:
Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin.
Lancet 1996; 348: 1049–54.
3. Northover J, Glynne-Jones R, Sebag-Montefiore D, et al.:
Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I).
Br J Cancer 2010; 102: 1123–8
4. Flam M, John M, Pajak TF, et al.:
Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study.
J Clin Oncol 1996; 14: 2527–39
5. Ajani JA, Winter KA, Gunderson LL, et al.:
Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial.
JAMA 2008; 299:1914–21
6. Gunderson LL, Winter KA, Ajani JA, et al.:
Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin.
J Clin Oncol 2012;30: 4344–51
7. James RD, Glynne-Jones R, Meadows HM, et al.:
Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial.
Lancet Oncol 2013; 14: 516–24
8. Peiffert D, Tournier-Rangeard L, Gérard JP, et al.:
Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial.
J Clin Oncol 2012; 30: 1941–8.
9.) Lukovic J, et al.:
Evaluation of dosimetric predictors of toxicity after IMRT with concurrent chemotherapy for anal cancer.
Radiotherapy and Oncology 2023;178:109429
