Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer:
Disease-Free Survival Results From a Randomized, Open-Label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial
Thierry Andr´e, Dewi Vernerey, Laurent Mineur, Jaafar Bennouna, J´er ˆ ome Desrame, Roger Faroux, Serge Fratte,
Marine Hug de Larauze, Sophie Paget-Bailly, Benoist Chibaudel, Jeremie Bez, J´er ˆ ome Dauba, Christophe Louvet,
C´eline Lepere, Olivier Dupuis, Yves Becouarn, May Mabro, Jo¨elle Egreteau, Olivier Bouche, Ga¨el Deplanque, Marc
Ychou, Marie Pierre Galais, François Ghiringhelli, Louis Marie Dourthe, Jean-Baptiste Bachet, Ahmed Khalil,
Franck Bonnetain † , Aimery de Gramont, and Julien Taieb for PRODIGE investigators, GERCOR, F´ed´eration
Française de Canc´erologie Digestive, and UNICANCER
In International Duration Evaluation of Adjuvant Chemotherapy (IDEA) France, as part of the IDEA international collaboration
3 and 6 months of modified FOLFOX6 (mFOLFOX6: infusional fluorouracil, leucovorin, and oxaliplatin) or
capecitabine plus oxaliplatin (CAPOX)
by physician choice.
2,010 eligible patients received either 3 or 6 months of chemotherapy (modified intention-to-treat population);
2,000 (99%) had stage III colon cancer (N1: 75%, N2: 25%);
1,809 (90%) received mFOLFOX6, and
201 (10%) received CAPOX.
The median age was 64 years, and the
median follow-up time was 4.3 years.
Overall, 94% (3 months) and 78% (6 months) of patients completed treatment ( fluoropyrimidines 6 oxaliplatin).
Maximal grade 2 and 3 neuropathy rates were
28% and 8% in the 3-month arm and
41% and 25% in the 6-month arm ( P , .001).
residual neuropathy greater than grade 1 were 3% in the 3-month arm and 7% in the 6-month arm( P , .001).
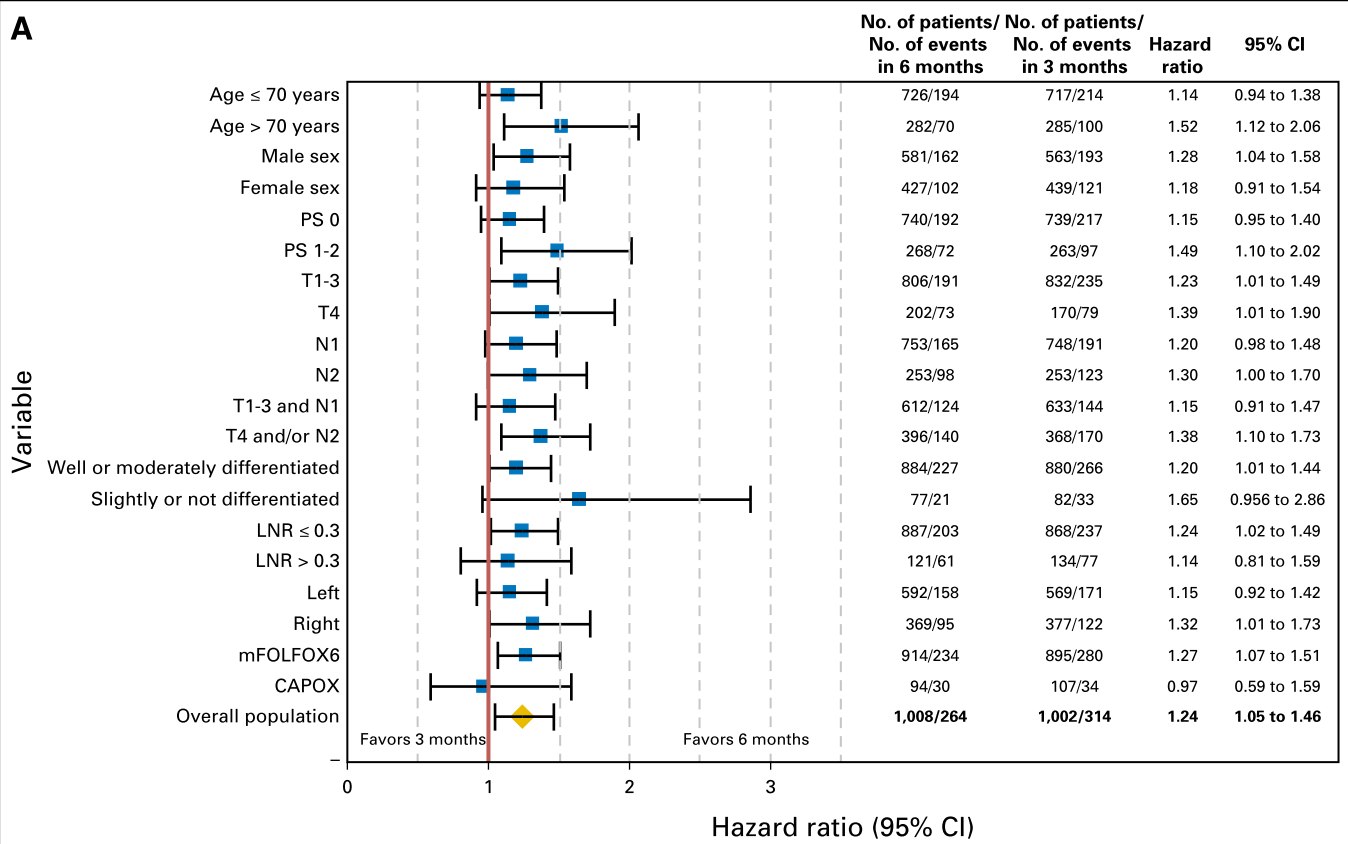
There were 578 DFS events:
314 and 264 in the 3- and 6-month arms, respectively.
3-year DFS rates were 72% and 76% in the 3- and 6-month arms, respectively (
hazard ratio [HR], 1.24; 95% CI, 1.05 to 1.46; P = .0112).
In the 3 and 6-month arms, respectively, for patients who received mFOLFOX6, the
3-year DFS rates were 72% and 76% (HR, 1.27; 95% CI, 1.07 to 1.51);
for the T4 and/or N2 population, they were 58% and 66% (HR, 1.44; 95% CI, 1.14 to 1.82); and for the
T1-3N1 population, they were 81% and 83% (HR, 1.15; 95% CI, 0.89 to 1.49).
Conclusion
IDEA France, in which 90% of patients received mFOLFOX6, shows superiority of 6 months of
adjuvant chemotherapy compared with 3 months, especially in the T4 and/or N2 subgroups. These
results should be considered alongside the international IDEA collaboration data.
J Clin Oncol 36:1469-1477. © 2018
DOI: https://doi.org/10.1200/JCO.2017.
76.0355
 |

