Fragestellung
Ergebnis
| Arm | Switch Exemesan | Tamoxifen | Differenz | p |
| OS / 8a | 352 /2294 | 405 /2305 | 2,4% | 0,04 |
Gesamtüberleben
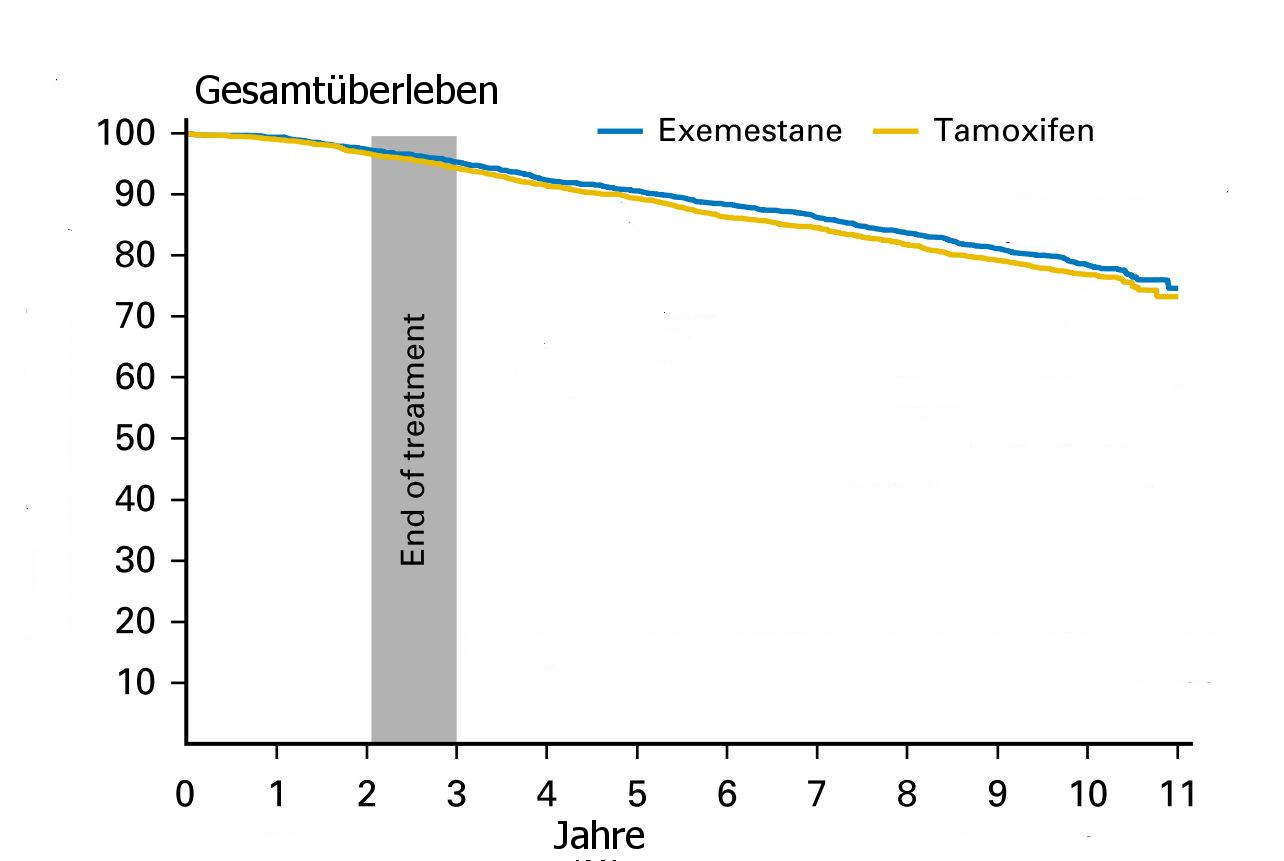
Nach Daten aus (1)
Wirkmechanismus
Einschluss
- Postmenopause
- abgeschlossene Primärtherapie
- 4599 Mamma-Ca-Patientinnen
Long-Term Follow-Up of the Intergroup Exemestane Study.
J Clin Oncol 2017;35:2507-2514
2.) Coombes RC, Hall E, Gibson LJ, et al:
A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer.
N Engl J Med 2004;350:1081-1092
3.) Coombes RC, Kilburn LS, Snowdon CF, et al:
Survival and safety of exemestane versus tamoxifen after 2-3 years tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial.
Lancet 2007;369:559-570
4.) Bliss JM, Kilburn LS, Coleman RE, et al:
Disease-related outcomes with long-term follow-up: An updated analysis of the intergroup exemestane study.
J Clin Oncol 2012;30:709-717
5.) Coleman RE, Banks LM, Girgis SI, et al:
Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): A randomised controlled study.
Lancet Oncol 2007;8:119-127
6.) Bertelli G, Hall E, Ireland E, et al:
Long-term endometrial effects in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): A randomised controlled trial of exemestane versus continued tamoxifen after 2-3 years tamoxifen.
Ann Oncol 2010;21:498-505
7.) Fallow fi eld LJ, Bliss JM, Porter LS, et al:
Quality of life in the intergroup exemestane study: A randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer.
J Clin Oncol 2006;24:910-917
8.) Fallow fi eld LJ, Kilburn LS, Langridge C, et al:
Long-term assessment of quality of life in the Intergroup Exemestane Study: 5 years post-randomisation.
Br J Cancer 2012;106:1062-1067
9.) Mieog JS, Morden JP, Bliss JM, et al:
Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2-3 years of tamoxifen: A retrospective analysis of the Intergroup Exemestane Study.
Lancet Oncol 2012;13:420-432
