zurück Home |
CLEOPATRA |
Allgemeines |
Clinical Evaluation of Pertuzumab and Trastuzumab |
|
We randomly assigned
808 patients with
HER2-positive
metastatic
breast cancer to
receive placebo plus trastuzumab plus docetaxel (control group) or
pertuzumab plus trastuzumab plus docetaxel (pertuzumab group) as
first-line treatment
until the time of disease progression or the development of toxic effects that could not be effectively managed.
The primary end point was independently assessed pro-
gression-free survival. Secondary end points included overall survival, progression-
free survival as assessed by the investigator, the objective response rate, and safety.
Results
The median progression-free survival was 12.4 months in the control group, as com-
pared with 18.5 months in the pertuzumab group (hazard ratio for progression or
death, 0.62; 95% confidence interval, 0.51 to 0.75; P<0.001). The interim analysis of
overall survival showed a strong trend in favor of pertuzumab plus trastuzumab plus
docetaxel. The safety profile was generally similar in the two groups, with no increase
in left ventricular systolic dysfunction; the rates of febrile neutropenia and diarrhea
of grade 3 or above were higher in the pertuzumab group than in the control group.
Conclusions
The combination of pertuzumab plus trastuzumab plus docetaxel, as compared with
placebo plus trastuzumab plus docetaxel, when used as first-line treatment for HER2-
positive metastatic breast cancer, significantly prolonged progression-free survival,
with no increase in cardiac toxic effects. (Funded by F. Hoffmann–La Roche/Genen-
tech; ClinicalTrials.gov number, NCT00567190.)
Fragestellung |
Verbessert Pertuzumab die first-line Therapie des metastasierten, Her2-positiven Mammakarzinoms mit Trastuzumab und Docetaxel? |
Antwort |
Die zusätzliche Gabe von Pertuzumab verlängert signifikant das progressionsfreie Überleben und das Gesamtüberleben bei einer first-line Therapie des metastasierten, Her2-positiven Mammakarzinoms mit Trastuzumab und Docetaxel! |
Behandlungs-Arm |
Pertuzumab + Trastuzumab + Docetaxel |
Kontroll - Arm |
Trastuzumab + Docetaxel |
Ergebnisse |
Endpunkt |
ohne Pertuzumab |
mit Pertuzumab |
Statistik |
PSF | alle (1) |
12,4 Monate |
18,5 Monate |
p < 0,0001 |
ohne Trastuzumab-Vorbehandlung (1) |
12,6 Monate |
21,6 Monate |
|
mit Trastuzumab-Vorbehandlung (1) |
10,4 Monate |
16,9 Monate | |
| OS (3) |
40,8 Monate |
56,5 Monate |
HR 0,68, p=0,0002 |
| PFS (3) |
|
besser |
HR 0,68, p = 0,0001 |
Follow up 50 Monate |
Gesamt-Überleben |
Beim metastasierten, her2-positiven Mammakarzinom kann mit Pertuzumab, Trastuzumab und Docetaxel ein längeres Gesamt-Überleben erzielt werden als mit Trastuzumab und Docetaxel.
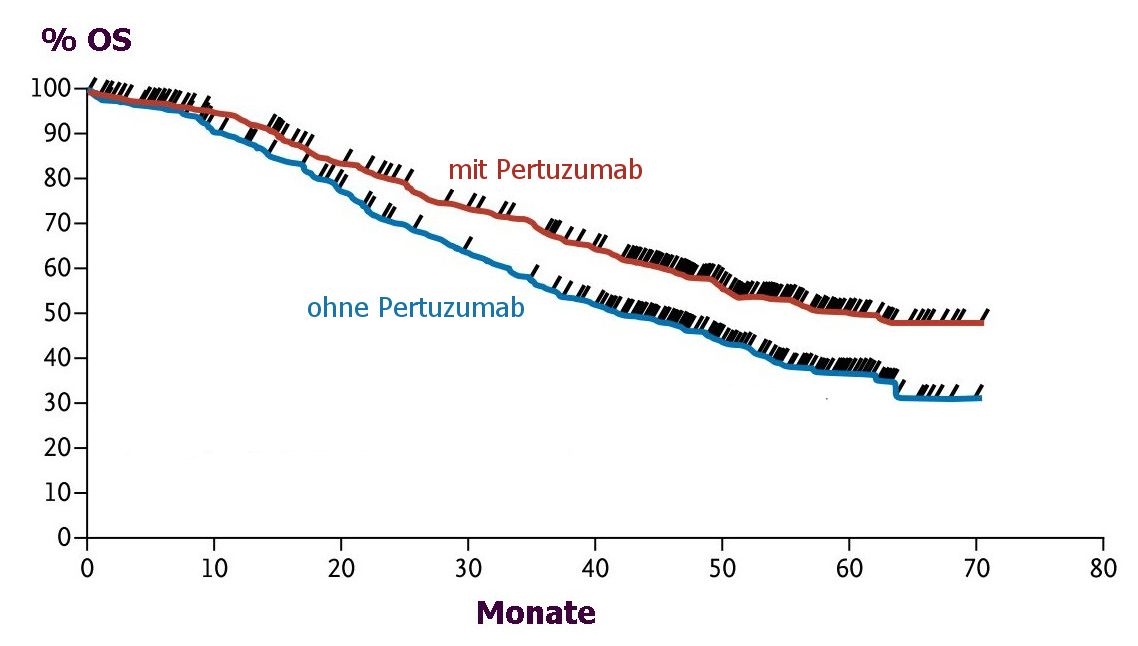 |
progressionsfreies Überleben |
Beim metastasierten, her2-positiven Mammakarzinom kann mit Pertuzumab, Trastuzumab und Docetaxel ein längeres progressionsfreies Überleben erzielt werden als mit Trastuzumab und Docetaxel.
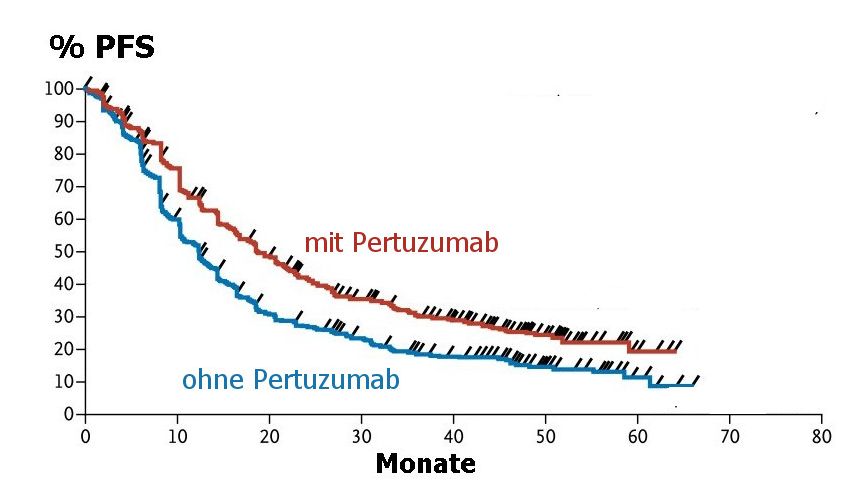
Modifiziert nach (1) |
Patienten |
- 808 Frauen mit Mammakarzinom
- lokal rezidiviert nicht resektabel oder
- Fernmetastasen
- Her2-positiv
|
Quellen |
1.) Baselga J, et al.:
Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer.
NEJM 366(2012):109-19
2.) JCO 31(2013) Supl Abstract 600
3.) Swain S, Kim S, Cortes J, et al.:
ESMO Congress 2014, 26.-30.9.2014, Madrid, Abstract 350O
4.) Swain SM, et al. for the CLEOPATRA Study Group:
Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer.
N Engl J Med 2015;372:724-34. doi: 10.1056/NEJMoa1413513.
|
Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer.
Sandra M. , for the CLEOPATRA Study Group
N Engl J Med 2015;372:724-34. DOI: 10.1056/NEJMoa1413513
Background
In patients with metastatic breast cancer that is positive for human epidermal
growth factor receptor 2 (HER2), progression-free survival was significantly im-
proved after first-line therapy with pertuzumab, trastuzumab, and docetaxel, as
compared with placebo, trastuzumab, and docetaxel. Overall survival was signifi-
cantly improved with pertuzumab in an interim analysis without the median being
reached. We report final prespecified overall survival results with a median follow-
up of 50 months.
Methods
We randomly assigned patients with metastatic breast cancer who had not received
previous chemotherapy or anti-HER2 therapy for their metastatic disease to receive
the pertuzumab combination or the placebo combination. The secondary end
points of overall survival, investigator-assessed progression-free survival, indepen-
dently assessed duration of response, and safety are reported. Sensitivity analyses
were adjusted for patients who crossed over from placebo to pertuzumab after the
interim analysis.
Results
The median overall survival was 56.5 months (95% confidence interval [CI], 49.3 to
not reached) in the group receiving the pertuzumab combination, as compared with
40.8 months (95% CI, 35.8 to 48.3) in the group receiving the placebo combination
(hazard ratio favoring the pertuzumab group, 0.68; 95% CI, 0.56 to 0.84; P<0.001),
a difference of 15.7 months. This analysis was not adjusted for crossover to the per-
tuzumab group and is therefore conservative. Results of sensitivity analyses after ad-
justment for crossover were consistent. Median progression-free survival as assessed
by investigators improved by 6.3 months in the pertuzumab group (hazard ratio, 0.68;
95% CI, 0.58 to 0.80). Pertuzumab extended the median duration of response by
7.7 months, as independently assessed. Most adverse events occurred during the admin-
istration of docetaxel in the two groups, with long-term cardiac safety maintained.
Conclusions
In patients with HER2-positive metastatic breast cancer, the addition of per tuzumab
to trastuzumab and docetaxel, as compared with the addition of placebo, signifi-
cantly improved the median overall survival to 56.5 months and extended the re-
sults of previous analyses showing the efficacy of this drug combination. (Funded
by F. Hoffmann–La Roche and Genentech; CLEOPATRA ClinicalTrials.gov number,
NCT00567190.)

|


