zurück
Home |
COMBI-AD - Studie |
| allgemeines |
In this double-blind, placebo-controlled, phase 3 trial, we randomly assigned 870
patients with completely resected, stage III melanoma with BRAF V600E or V600K
mutations to receive oral dabrafenib at a dose of 150 mg twice daily plus trametinib at
a dose of 2 mg once daily (combination therapy, 438 patients) or two matched placebo
tablets (432 patients) for 12 months. The primary end point was relapse-free survival.
Secondary end points included overall survival, distant metastasis–free survival,
freedom from relapse, and safety.
RESULTS
At a median follow-up of 2.8 years, the estimated 3-year rate of relapse-free survival
was 58% in the combination-therapy group and 39% in the placebo group (hazard
ratio for relapse or death, 0.47; 95% confidence interval [CI], 0.39 to 0.58; P<0.001).
The 3-year overall survival rate was 86% in the combination-therapy group and 77%
in the placebo group (hazard ratio for death, 0.57; 95% CI, 0.42 to 0.79; P = 0.0006),
but this level of improvement did not cross the prespecified interim analysis boundary
of P=0.000019. Rates of distant metastasis–free survival and freedom from relapse
were also higher in the combination-therapy group than in the placebo group. The
safety profile of dabrafenib plus trametinib was consistent with that observed with
the combination in patients with metastatic melanoma.
CONCLUSIONS
Adjuvant use of combination therapy with dabrafenib plus trametinib resulted in a
significantly lower risk of recurrence in patients with stage III melanoma with BRAF
V600E or V600K mutations than the adjuvant use of placebo and was not associated
with new toxic effects. (Funded by GlaxoSmithKline and Novartis; COMBI-AD
ClinicalTrials.gov, NCT01682083; EudraCT number, 2012-001266-15.) |
| Fragestellung |
Verbessert eine adjuvante Kombination des MEK-IH Trametinib
mit dem BRAF-IH Dabrafenib das
Behandlungsergebnis des komplett resezierten Melanoms mit
BRAF-V600-Mutation im Stadium III? |
| Antwort |
Eine adjuvante Kombination des MEK-IH Trametinib
mit dem BRAF-IH Dabrafenib verbessert das Gesamtüberleben und das
rezidivfreie Überleben des komplett resezierten Melanoms mit
BRAF-V600-Mutation im Stadium III hoch signifikant! |
Mechanismus |
Dabrafenib ist ein BRAF-IH |
Trametinib ist ein
MEK-IH |
| Ergebnis |
| Arm |
Dabrafenib plus Trametinib |
Placebo |
Bemerkungen |
| Patienten |
438 |
432 |
|
| 3a-RFS |
58% |
39% |
HR 0,47; 95%-CI 0,39–0,58; p < 0,001 |
| 3a-OS |
86% |
77% |
HR 0,57; 95%-CI 0,42–0,79; p = 0,0006 |
| Pyrexie |
63% |
11% |
|
| Übelkeit |
40% |
20% |
|
| Diarrhoe |
33% |
15% |
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
|
| RFS |
Das rezidivfreie Überleben von BRAF-V600-mutierten Melanomen
im Stadium III ist mit einer postoperativen Therapie mit kombinierter BRAF- und MEK-Blockade
hoch signifikant besser.
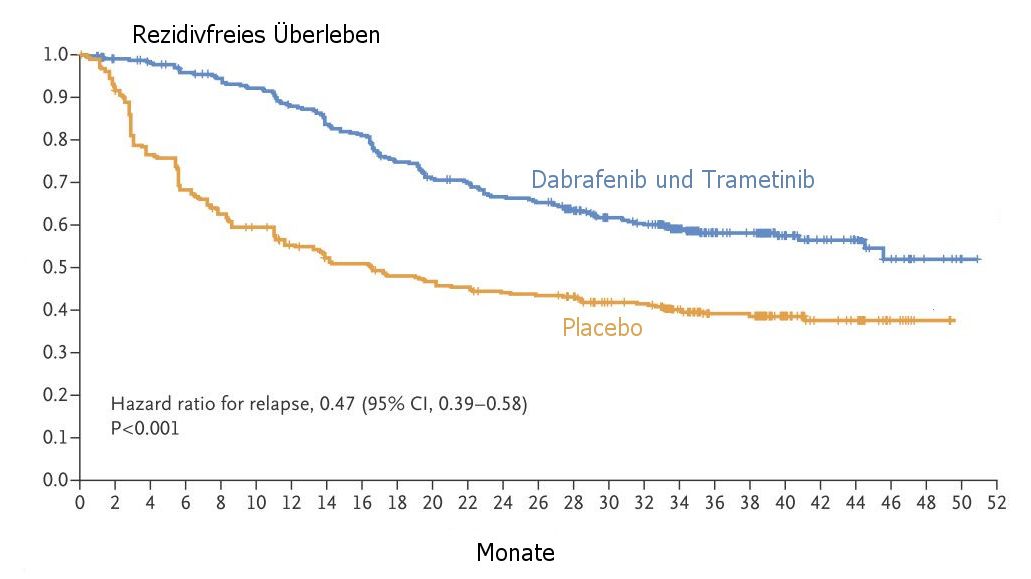
Modifiziert nach Daten aus (1) |
| OS |
Die Überlebensrate von BRAF-V600-mutierten Melanomen
im Stadium III ist mit einer postoperativen Therapie mit kombinierter BRAF- und MEK-Blockade
hoch signifikant besser.
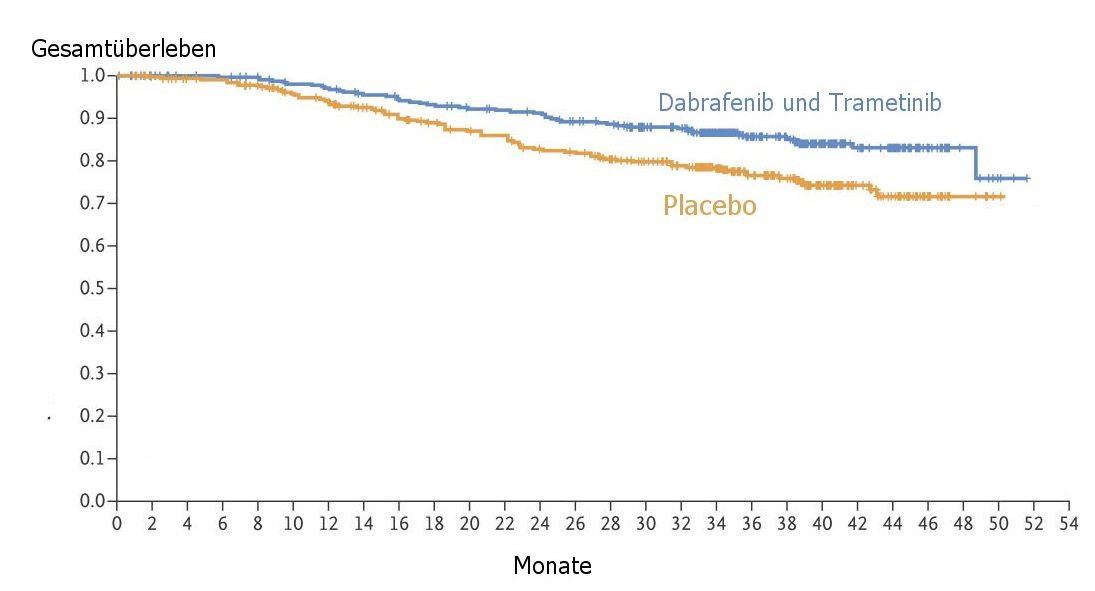
Modifiziert nach Daten aus (1) |
| Nebenwirkungen |
|
| Studie | Phase III |
Random Dabrafenib + Trametinib 12 Monate versus
Placebo |
|
| Patienten |
- 870 Melanompatienten
- komplett reseziert
- Stadium III
- keine systemische Vorbehandlung
- BRAF-V600-Mutation
- 2013 - 2014 aus 26 Ländern
|
| Applikation |
Dabrafenib oral 2 x 150 mg/d |
Trametinib oral 2 mg/d
|
| Quellen |
1.) Long GV, et al.:
Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma.
N Engl J Med 2017;377:1813-23
DOI: 10.1056/NEJMoa1708539
2.)
ClinicalTrials.gov: NCT01682083
3.)
EudraCT number: 2012-001266-15.
|
 |


