| zurück
Home |
GLARIUS - Studie |
| Fragestellung |
|
| Purpose
In patients with newly diagnosed glioblastoma that harbors a nonmethylated O
6
-methylguanine –
DNA methyltransferase promotor, standard temozolomide (TMZ) has, at best, limited ef fi cacy. The
GLARIUS trial thus explored bevacizumab plus irinotecan (BEV+IRI) as an alternative to TMZ.
Patients and Methods
In this phase II, unblinded trial 182 patients in 22 centers were randomly assigned 2:1 to BEV (10 mg/kg
every 2 weeks) during radiotherapy (RT) followed by maintenance BEV (10 mg/kg every 2 weeks) plus
IRI(125 mg/m
2
every 2 weeks) or to daily TMZ (75 mg/m
2
) during RT followed by six courses of TMZ
(150-200 mg/m
2
/d for 5 days every 4 weeks). The primary end point was the progression-free survival
rate after 6 months (PFS-6).
Results
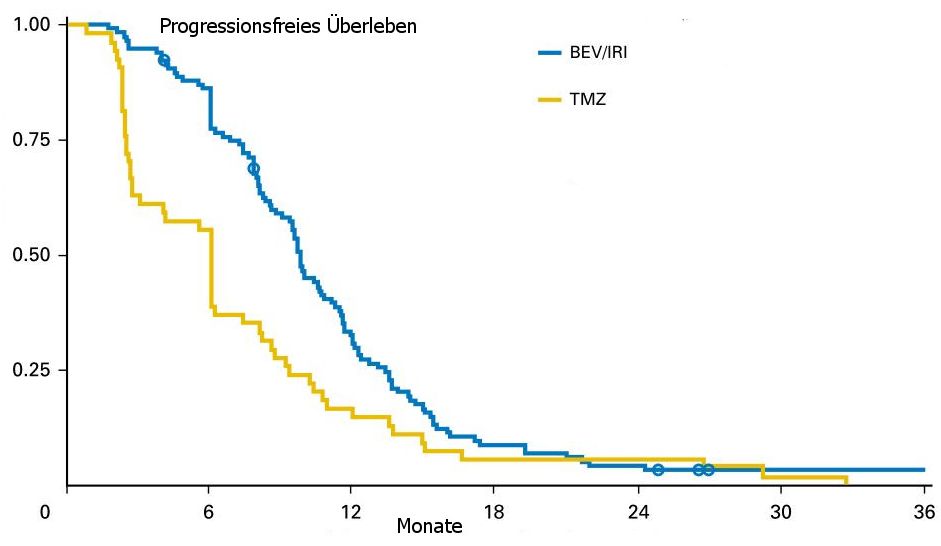
In the modi fi ed intention-to-treat (ITT) population, PFS-6 was increased from 42.6% with TMZ (95% CI,
29.4% to 55.8%) to 79.3% with BEV+IRI (95% CI, 71.9% to 86.7%; P , .001). PFS was prolonged
from a median of 5.99 months (95% CI, 2.7 to 7.3 months) to 9.7 months (95% CI, 8.7 to 10.8 months;
P , .001). At progression, crossover BEV therapy was given to 81.8% of all patients who received any
sort of second-line therapy in the TMZ arm. Overall survival (OS) was not different in the two arms: the
median OS was 16.6 months (95% CI, 15.4 to 18.4 months) with BEV+IRI and was 17.5 months (95%
CI, 15.1 to 20.5 months) with TMZ. The time course of quality of life (QOL) in six selected domains of
the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire (QLQ)
– C30 and QLQ-BN20 (which included cognitive functioning), of the Karnofsky performance score, and
of the Mini Mental State Examination score was not different between the treatment arms.
Conclusion
BEV+IRI resulted in a superior PFS-6 rate and median PFS compared with TMZ. However, BEV+IRI did not
improve OS, potentially because of the high crossover rate. BEV+IRI did not alter QOL compared with TMZ.
|
| Ergebnis |
nicht methylierter MGMT-Promotor: Bevacizumab + Irinotecan
besser als Temozolomid |
| Ereignis |
Beva + Irino |
Temozolomid |
Statistik |
| 6-Monate PFS |
71,1% |
26,2% |
p<0,001 |
| Patienten |
116 |
54 |
|
| OS |
16,6 Monate |
14,8 Monate |
p<0,031 |
|
| PFS |
 |
| Patienten |
- 170
- Glioblastom
- nicht methylierter MGMT-Promotor
- Alter 56a median
- 50% komplette Resektion
- 80% KI>90
|
| Standardarm |
RT + Temozolomid75mg/qm täglich, dann 6 Zyklen Temozolomid 150-200mg/qm d1-5 q29 |
| Vergleichsarm |
Bevacizumab 10mg/kg d1 q15 + Irinothecan 125mg/qm d1 q15 |
Teil von |
Glioblastom-Studien |
Glioblastom |
High-Grade-Gliome |
Gliome |
ZNS-Tumore |
| Quelle |
1.) Herrlinger U, et al.:
Bevacizumab, irinotecan, and radiotherapy versus standard temozolomid and radiotherapy in newly diagnose, MGMT - nonmethylated glioblastoma patients:
First results from the randomized multicenter GLARIUS trial.
JCO 31(2013) suppl. Abst LBA2000
2.) Herrlinger U, Schäfer N, Steinbach J, et al.:
Bevacizumab plus irinotecan versus temozolmide in a newly diagnosed
O6-methylguanine-DNA methyl-transferase nonmethylated glioblastoma: The
randomized GLARIUS Trial.
J Clin Oncol 2016;34:1611-1619
|
 | |

