High-Grade-Gliome
Allgemeines
High Grade Gliome
| Histologie | SEER 1998-2007 | OS/Monate | |
| Glioblastom | 12.115 | 83,8% | 10 |
| anaplastisches Astrozytom | 1312 | 9,1% | 30 |
| anaplastisches Oligodendrogliom | 718 | 4,9% | 64 |
| gemischtes anaplastisches Oligoastrozytom | 316 | 2,2% | 67 |
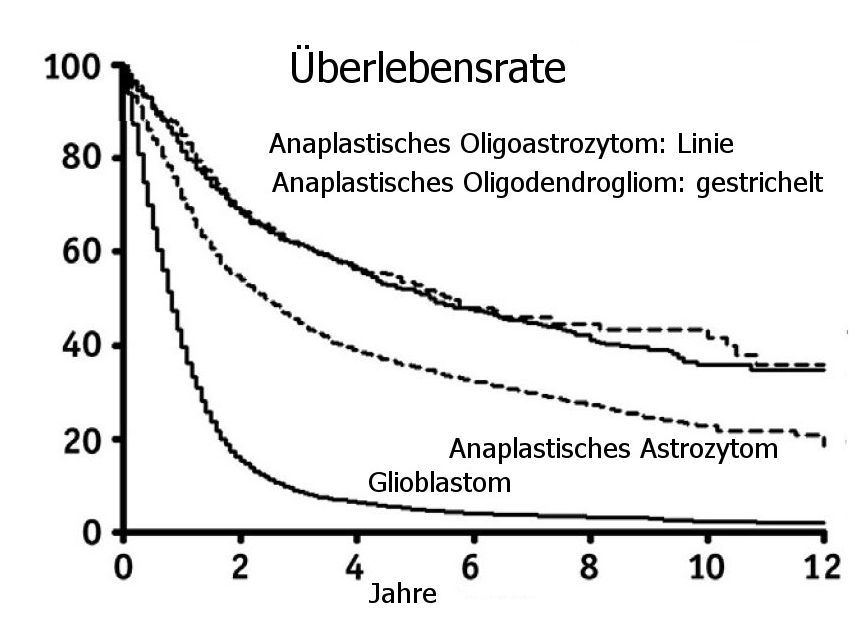 Modifiziert nach (1). SEER-Analyse.
Modifiziert nach (1). SEER-Analyse.molekulare Stratifizierung
diffuse Gliome
WHO-Gad II-IV
| IDH1 oder IDH2 mutiert | IDH1 und IDH2 Wildtyp | ||
| 1p/19q-Kodeletion | 1p/19q intakt | TERT-Promotor mutiert | 10 |
| TERT-Promotor mutiert | ATRX-Mutation | + Chr 7 / - Chr. 10 | 30 |
| CIC -Mutation | TP53-Mutation | EGFR-Amplifikation | 64 |
| Oligogendrogliome WHO-Grad II und III | Astrozytäre Gliome WHO-Grad II und III (IV?) | Glioblastom WHO IV (Anaplastische Astrozytom Grad III) |
67 |
| günstige Prognose | Intermediäre Prognose | Ungünstige Prognose | |
Modifiziert nach (1). SEER-Analyse.
| Response | Kriterien |
| Complete response |
|
| Partial response |
|
| Stable disease | Nicht ausreichend Kriterien für kompletten Response oder Progression |
| Progression |
|
| Kriterium | Complete response | Partial response | Stable disease | Progression |
| T1 gadolinium enhancing disease | keine | ≥ 50% Abnahme | ≥ 25% bis 50% Abnahme | ≥ 25% Zunahme |
| T2/FLAIR | gleich oder Abnahme | gleich oder Abnahme | gleich oder Abnahme | Zunahme |
| New lesion | Nein | Nein | Nein | Ja |
| Cortisonbedarf | kein | gleich oder Abnahme | gleich oder Abnahme | - |
| Klinischer Status | Gleich oder besser | Gleich oder besser | Gleich oder besser | Verschlechterung- |
| Erforderliche Kriterien | alle | alle | alle | irgendeines |
| Intraoperativ werden Wafer mit Chemotherapeutika in die Wundhöhle eingebracht. | Es werden abbaubare Carmustin - haltige Wafer verwendet. | Die Wafer enthalten 3,85% Carmustin. Die Freisetzung erfolgt in 2 - 3 Wochen. | |
| Bis zu 8 Wafer werden in die Wundhöhle eingebracht. | 7,7 mg Carmustin enthält ein Wafer | ||
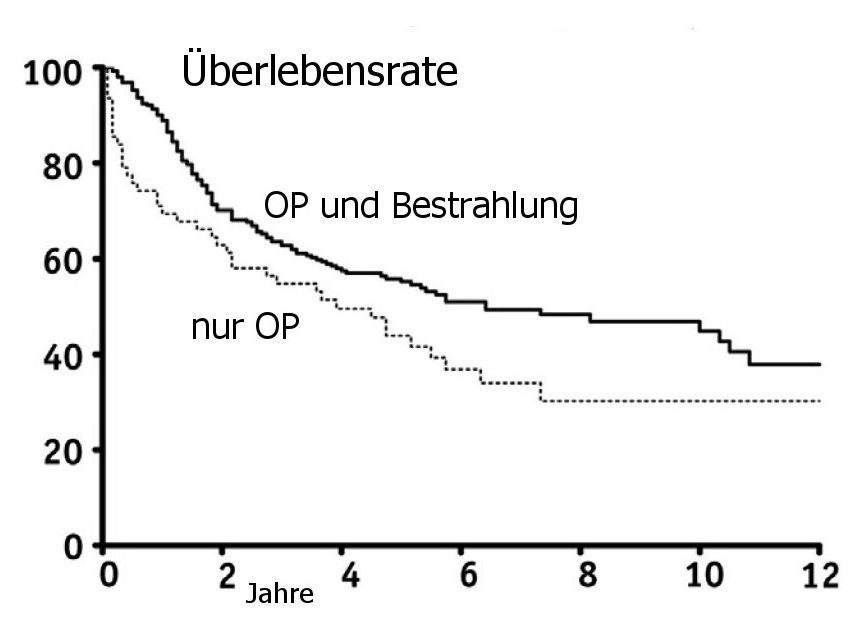
| 2 Randomisierte Studien wurden durchgeführt. | Beide finden ein längeres Überleben mit Wafern. | Das Höchstalter war 65a. | |
| 1. Studie (3) | Wurde geschlossen wegen Mangel an Wafern. | 32 Patienten mit High-Grade Gliomen. | Das Gesamtüberleben war in der Wafer-Gruppe bessser: 58,1 Wochen versus 39,9 Wochen, p = 0,012. |
| 2. Studie (4,5) | Multizentrische randomisierte Studie mit 240 Patienten, davon 207 GB. | Postoperative Rradiotherapie mit 55–60 Gy. | |
| Keine systemische Therapy bis zum Rezidiv außer bei anaplastischem Oligodendrogliomas (n = 9). | Das Gesamtüberleben war in der Wafer-Gruppe bessser: 13,8 Monate versus 11,6 Monate. HR = 0,73; 95%-CI: 0,56–0,96; P = 0,017. | ||
| systematischer Review (6) | Auswertung beider Studien. | HR für Gesamtüberleben 0.65; 95% - CI = 0,48–0,86; P = 0,003. | |
Teil von
The Impact of Adjuvant Radiation Therapy for High-Grade Gliomas by Histology in the United States Population.
Int J Radiation Oncol Biol Phys 90(2014): 894-902
2.) Bleehen NM, Stenning SP:
A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma.
The Medical Research Council Brain Tumour Working Party.
Br J Cancer 1991;64):769-74
3.) Valtonen S, Timonen U, Toivanen P, et al.:
Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study.
Neurosurgery 1997;41: 44-8
4.) Westphal M, Hilt DC, Bortey E, et al.:
A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma.
Neuro-oncol 2003;5):79-88
5.) Westphal M, Ram Z, Riddle V, et al.:
Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial.
Acta Neurochir (Wien) 2006;148: 269-75
6.) Hart MG, Grant R, Garside R, et al.:
Chemotherapeutic wafers for high grade glioma.
Cochrane Database Syst Rev 2008;CD007294
7.) Macdonald D, Cascino T, Schold SJ, et al:
Response criteria for phase II studies of supratentorial malignant glioma.
J Clin Oncol 1990;8:1277-1280
8.) Wen PY, Macdonald DR, Reardon DA, et al.:
Updated response assessment criteria for highgrade gliomas: Response Assessment in NeuroOncology Working Group.
J Clin Oncol 2010; 28: 196372.
9.)Dejonckheere C S, et al.: Chasing a rarity: a retrospective single-center evaluation of prognostic factors in primary gliosarcoma.
doi: 10.1007/s00066-021-01884-0
Strahlenther Onkol 2022;198:468–474
10.) Baumert B G, et al.:
ESTRO-EANO guideline on target delineation and radiotherapy for IDH-mutant WHO CNS grade 2 and 3 diffuse glioma.
Radiotherapy and Oncology 202 (2025) 110594
